Water Activity
Also known as aw or water availability
What is Water Activity?
Water activity, also known as aw, is an intrinsic food property which refers to the amount of free water that is available to participate in chemical or biological processes, either as catalyst or just as a medium in which molecules easily flow.
Along with osmotic pressure and pH, water activity is a critical parameter to establish:
- The shelf life of foods
- How long food products last before undergoing microbial spoilage
How it works
The earliest measurements of aw involved manometric determinations of vapor pressures and dividing the vapor pressure of a solution or food product (p1) by the vapor pressure of pure water (p0). The ideal aw of a solution or food, with the solvent being merely water, can be determined by dividing the moles of solvent (n2) by the sum of the moles of solvent and the moles of solute (n1).1

For example, the addition of 1 mole of sugar to 1 L (55.5 moles) of water would give a 1.0 molal solution with an ideal aw value of 55.5 / (1 + 55.5) = 0.982.
Solutes in food can be sugars (both mono- and disaccharides), salt and organic acids. Ketchup, for example, can be seen as a water solution in which solutes are diluted or dissolved. Of course, solid food products, such as bread or cakes, do not only contain solutes but also plasticized polysaccharides and proteins which confer on baked goods their unique texture.
Nowaday, this activity can be accurately measured by using reliable instruments that measure the equilibrium relative humidity (ERH) of food samples. The aw of a food is simply its ERH / 100. Because ERH readings are limited to the range of 0% to 100%, aw readings will range from 0 to 1.0. In modern laboratories, aw determinations are made quickly and accurately by the instrumental determination of dew point depressions.1
Difference between moisture content and water activity
Water activity should not be confused with moisture. Moisture content of foods is a quantitative measure of how much total water, both “bound” and “free”, a food product contains. On the other hand, water activity is rather a qualitative measure of the “free water” content of a food sample. Bound water simply means the water molecules which are linked to sugars, organic acids, salts and other small molecules by polar or hydrogen bonding.
Application
Water activity values of selected food products:
| Food Ingredient | aw value |
|---|---|
| Water | 1.00 |
| Fresh meat, poultry and seafood | 0.99 |
| Pan bread (fresh) | 0.93 |
| Batter Cakes | 0.81 |
| Icings, frostings, and glazes | 0.78 |
| Dried fruit | 0.65 – 0.75 |
| Corn syrup | 0.70 |
| Wheat flour | 0.65 |
| Dry pasta | 0.1 – 0.40 |
| Soda crackers | 0.30 |
It can be concluded from the table that the lower the moisture content, the lower the activity. This rule of course applies when water is the only component that changes, meaning that solids are concentrated.
Despite being liquid and having a much higher moisture content, syrups have lower aw values than solid food products, such as cakes or bread. This is because syrups have much higher amounts of sugars and salts added to them, causing water in such systems to be “more bound” or “less free” than in bread matrices.
Water activity and shelf-life of food products
Intrinsic or inherent food properties, such as water activity and pH, can be controlled to assure food safety, as well as to inhibit microbiological spoilage and to protect product quality.
Along with thermal technologies, salting, drying, acidification, and fermentation, the reduction of water activity in food enables bakers to extend shelf life and become more efficient.
As a rule of thumb, the higher the aw, the shorter the shelf life of the food product (the less stable it becomes).
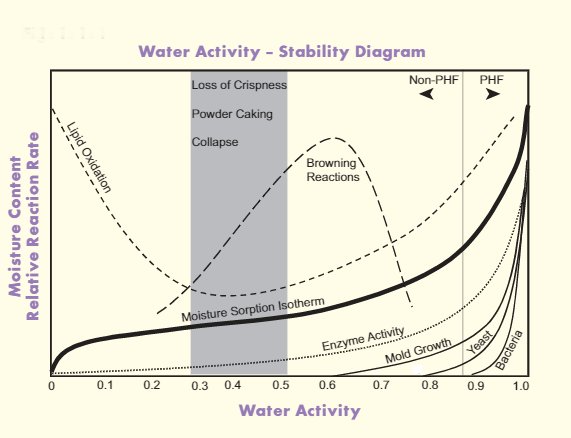
How does water activity impact shelf life?
References
- Barbosa‐Cánovas, G.V., Fontana Jr., A.J., Schmidt, S.J., and Labuza, T.P. Water Activity in Foods: Fundamentals and Applications, 2nd edition, John Wiley & Sons, Inc., 2020.
- “Water Activity (aw) in Foods.” U.S. Food and Drug Administration, 27 Jan. 2015. www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnicalGuides/ucm072916.htm. Accessed 02 Feb. 2021.
- “Fundamentals of Water Activity.” Pullman, Washington: Decagon Device, 2006. http://nfscfaculty.tamu.edu/talcott/courses/FSTC605/Papers%20Reviewed/Fundamentals%20of%20Aw-Decagon.pdf. Accessed 02 Feb. 2021.
- “Evaluation and Definition of Potentially Hazardous Foods – Chapter 3. Factors That Influence Microbial Growth.” U.S. Food and Drug Administration, 19 Mar. 2015. www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm094145.htm. Accessed 03 Feb. 2021.
- Slade, Louise, Harry Levine, and David S. Reid. ” Beyond Water Activity: Recent Advances Based on an Alternative Approach to the Assessment of Food Quality and Safety.” Critical Reviews in Food Science and Nutrition 30.2-3 (1991): 115-360.

